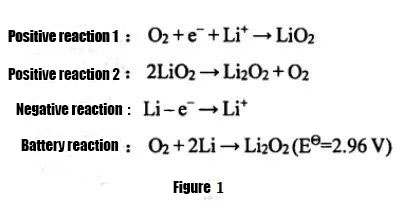01 Yintoni iibhetri ze-lithium-moya kunye neebhetri ze-lithium-sulphur?
① Li-air ibhetri
Ibhetri ye-lithium-moya isebenzisa i-oksijini njenge-electrode efanelekileyo ephendulayo kunye ne-metal lithium njenge-electrode engafanelekanga.Inomthamo ophezulu we-theoretical energy (3500wh / kg), kwaye amandla ayo okwenene angafikelela kwi-500-1000wh / kg, ephezulu kakhulu kunenkqubo yebhetri ye-lithium-ion eqhelekileyo.Iibhetri ze-lithium zomoya zenziwe ngee-electrode ezilungileyo, i-electrolytes kunye ne-electrode engalunganga.Kwiinkqubo zebhetri ezingenamanzi, i-oksijini ecocekileyo ngoku isetyenziswa njengegesi yokusabela, ngoko ke iibhetri ze-lithium-air zingabizwa ngokuba yi-lithium-oxygen ibhetri.
Ngowe-1996, uAbraham et al.ihlanganise ngempumelelo ibhetri yokuqala engenamanzi ye-lithium-moya kwilabhoratri.Emva koko abaphandi baqala ukunikela ingqalelo kwi-electrochemical reaction yangaphakathi kunye nendlela yeebhetri ze-lithium-air ezingenamanzi;ngo-2002, Funda et al.yafumanisa ukuba ukusebenza kwe-electrochemical yeebhetri ze-lithium-air kuxhomekeke kwi-solvent ye-electrolyte kunye ne-air cathode materials;ngo-2006, Ogasawara et al.isetyenziswe i-Mass spectrometer, yabonakaliswa okokuqala ukuba i-Li2O2 i-oxidized kwaye i-oksijini yakhululwa ngexesha lokutshaja, eqinisekisa ukuguqulwa kwe-electrochemical ye-Li2O2.Ngoko ke, iibhetri ze-lithium-air zifumene ingqalelo eninzi kunye nophuhliso olukhawulezayo.
② Ibhetri yeLithium-sulphur
Ibhetri ye-lithium-sulphur yinkqubo yebhetri yesibini esekelwe kwimpendulo eguqulwayo yomthamo ophezulu wesulfure (1675mAh / g) kunye nesinyithi se-lithium (3860mAh / g), kunye ne-avareji yokukhupha umbane malunga ne-2.15V.Uxinaniso lwamandla olwazi lungafikelela kuma-2600wh/kg.Izinto zayo eziluhlaza zineenzuzo zexabiso eliphantsi kunye nobuhlobo bendalo, ngoko inamandla amakhulu ophuhliso.Ukuveliswa kweebhetri ze-lithium-sulfur kunokulandelwa emva kwe-1960, xa u-Herbert no-Ulam bafaka isicelo se-patent yebhetri.Iprototype yale lithium-sulfur ibhetri isetyenziswe i-lithium okanye i-lithium alloy njengento engafanelekanga ye-electrode, isulfure njengento efanelekileyo ye-electrode kunye ne-aliphatic saturated amines.ye-electrolyte.Kwiminyaka embalwa kamva, iibhetri ze-lithium-sulfur zaphuculwa ngokuzisa i-solvents eziphilayo ezifana ne-PC, i-DMSO, kunye ne-DMF, kunye neebhetri ze-2.35-2.5V zafunyanwa.Ngasekupheleni kwee-1980s, ii-ethers zangqinwa ukuba ziluncedo kwiibhetri ze-lithium-sulphur.Kwizifundo ezilandelayo, ukufunyanwa kwe-ether-based electrolytes, ukusetyenziswa kwe-LiNO3 njenge-additive electrolyte, kunye nesiphakamiso se-carbon / sulfur composite positive electrode ziye zavula uphando lweebhetri ze-lithium-sulfur.
02 Umgaqo osebenzayo webhetri ye-lithium-air kunye nebhetri ye-lithium-sulfur
① Li-air ibhetri
Ngokweemeko ezahlukeneyo ze-electrolyte esetyenzisiweyo, iibhetri ze-lithium-air zingahlulwa zibe ziinkqubo zamanzi, iinkqubo eziphilayo, iinkqubo ezixutywe ngamanzi, kunye neebhetri ze-lithium zomoya.Phakathi kwazo, ngenxa yomthamo ophantsi webhetri ye-lithium-moya usebenzisa i-electrolyte esekwe emanzini, ubunzima ekukhuseleni isinyithi se-lithium, kunye nokuguqulwa okungahambi kakuhle kwenkqubo, iibhetri ze-lithium-air ezingenamanzi kunye ne-lithium-moya yonke. iibhetri zisetyenziswa kakhulu ngoku.Uphando.Iibhetri ze-lithium-air ezingenamanzi zaqala ukucetywa ngu-Abraham no-Z.Jiang kwi-1996. I-equation reaction equation iboniswe kuMzobo 1. Ukutshatyalaliswa kokutshaja kuchasene.I-electrolyte ikakhulu isebenzisa i-electrolyte ye-organic okanye i-electrolyte eqinile, kwaye imveliso yokukhutshwa ngokuyinhloko i-Li2O2, imveliso ayinakunyibilika kwi-electrolyte, kwaye kulula ukuqokelela kwi-electrode epholileyo yomoya, echaphazela amandla okukhutshwa kwebhetri ye-lithium-air.
Iibhetri zomoya zeLithium zineengenelo zokuxinana kwamandla aphezulu kakhulu, ubuhlobo bendalo, kunye nexabiso eliphantsi, kodwa uphando lwabo lusekutsha, kwaye kusekho iingxaki ezininzi ekufuneka zisonjululwe, ezifana ne-catalysis yokusabela kokunciphisa ioksijini, i-oxygen permeability kunye ne-hydrophobicity ye-electrodes yomoya, kunye nokuvalwa kwee-electrodes zomoya njl.
② Ibhetri yeLithium-sulphur
Iibhetri ze-lithium-sulphur ikakhulu zisebenzisa i-elemental sulfur okanye i-sulphur-based compounds njengezinto eziphathekayo ze-electrode yebhetri, kunye ne-metallic lithium isetyenziselwa ikakhulu i-electrode engalunganga.Ngethuba lenkqubo yokukhupha, i-lithium yensimbi efumaneka kwi-electrode engafanelekanga i-oxidized ukulahlekelwa i-electron kwaye ivelise i-lithium ion;emva koko ii-electron zidluliselwa kwi-electrode efanelekileyo ngokusebenzisa isiphaluka sangaphandle, kwaye i-lithium ion eyenziwe iphinde idluliselwe kwi-electrode efanelekileyo nge-electrolyte ukusabela ngesulfure ukwenza i-polysulfide.I-Lithium (i-LiPSs), kwaye emva koko iphendule ngakumbi ukuvelisa i-lithium sulfide ukugqiba inkqubo yokukhupha.Ngexesha lenkqubo yokutshaja, ii-ion ze-lithium kwii-LiPS zibuyela kwi-electrode engalunganga nge-electrolyte, ngelixa ii-electron zibuyela kwi-electrode engalunganga ngesekethe yangaphandle ukuze zenze isinyithi se-lithium kunye nee-ion ze-lithium, kwaye ii-LiPS ziyancitshiswa zibe yisulfure kwi-electrode elungileyo ukugqiba inkqubo yokutshaja.
Inkqubo yokukhutshwa kweebhetri ze-lithium-sulfur ubukhulu becala yi-multi-step, multi-electron, multi-phase complex complex reaction electrochemical reaction on the sulfur cathode, kunye ne-LiPSs ezinobude obuhlukeneyo be-chain ziguqulwa zibe enye kwenye ngexesha lenkqubo yokukhutshwa kwentlawulo.Ngethuba lenkqubo yokukhupha, ukusabela okunokuthi kwenzeke kwi-electrode efanelekileyo kuboniswe kuMfanekiso 2, kwaye ukusabela kwi-electrode engalunganga kuboniswe kuMfanekiso 3.
Izibonelelo zeebhetri ze-lithium-sulfur zicacile kakhulu, ezifana nomthamo ophezulu kakhulu wethiyori;akukho oksijini kwizinto eziphathekayo, kwaye i-oxygen evolution reaction ayiyi kwenzeka, ngoko ukusebenza kokhuseleko kulungile;izixhobo zesulfure zininzi kwaye i-elemental sulfure inexabiso eliphantsi;ihambelana nokusingqongileyo kwaye inetyhefu ephantsi.Nangona kunjalo, iibhetri ze-lithium-sulfur nazo zineengxaki ezinzima, ezifana ne-lithium polysulfide shuttle effect;i-insulation of elemental sulfure kunye neemveliso zayo zokukhupha;ingxaki yokutshintsha umthamo omkhulu;i-SEI engazinzanga kunye neengxaki zokhuseleko ezibangelwa yi-lithium anodes;into yokuzikhupha, njl.
Njengesizukulwana esitsha senkqubo yebhetri yesibini, iibhetri ze-lithium-moya kunye neebhetri ze-lithium-sulfur zinexabiso eliphezulu kakhulu lethiyori ethile, kwaye zitsale ingqalelo ebanzi kubaphandi kunye nemarike yebhetri yesibini.Okwangoku, ezi bhetri zimbini zisajongene neengxaki ezininzi zenzululwazi kunye nezobugcisa.Bakwinqanaba lokuqala lophando lophuhliso lwebhetri.Ukongeza kumthamo othile kunye nozinzo lwemathiriyeli yecathode yebhetri efuna ukuphuculwa ngakumbi, imiba ephambili efana nokhuseleko lwebhetri nayo ifuna ukusonjululwa ngokukhawuleza.Kwixesha elizayo, ezi ntlobo zimbini zintsha zeebhetri zisafuna ukuphuculwa kobugcisa obuqhubekayo ukuze kupheliswe iziphene zazo ukuze kuvuleke amathuba abanzi okusebenza.
Ixesha lokuposa: Apr-07-2023